Uranium (U) - Atomic, Physical & Chemical Properties, Uses, and Periodic Table Trends
Uranium Element Information, Facts, Properties, Trends, Uses, Comparison with other elements

Uranium (U) - Comprehensive Element Profile, Properties & Uses
In this comprehensive guide, you'll learn about Uranium's unique chemical and physical properties, trends in the periodic table, isotopes, and its historical significance. We'll also cover its abundance, crystal structure, electron configuration, and health & safety guidelines. Explore how Uranium compares with other elements and discover its many uses.
Uranium is a chemical element with symbol U and atomic number 92. It is a silvery-white metal in the Actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons.
Uranium is part of Actinide series. You can also download Printable Periodic Table of Elements Flashcards for Uranium in a PDF format.
Uranium Facts
Read key information and facts about element Uranium
| Name | Uranium |
| Atomic Number | 92 |
| Atomic Symbol | U |
| Atomic Weight | 238.02891 |
| Phase | Solid |
| Color | Silver |
| Appearance | - |
| Classification | Actinide |
| Natural Occurance | Primordial |
| Group in Periodic Table | - |
| Group Name | |
| Period in Periodic Table | period 7 |
| Block in Periodic Table | f-block |
| Electronic Configuration | [Rn] 5f3 6d1 7s2 |
| Electronic Shell Structure (Electrons per shell) | 2, 8, 18, 32, 21, 9, 2 |
| Melting Point | 1408 K |
| Boiling Point | 4200 K |
| CAS Number | CAS7440-61-1 |
How to Locate Uranium on Periodic Table
Periodic table is arranged by atomic number, number of protons in the nucleus which is same as number of electrons. The atomic number increases from left to right. Periodic table starts at top left ( Atomic number 1) and ends at bottom right (atomic number 118). Therefore you can directly look for atomic number 92 to find Uranium on periodic table.
Another way to read periodic table and locate an element is by using group number (column) and period number (row). To locate Uranium on periodic table look for cross section of group - and period 7 in the modern periodic table.
Uranium History
The element Uranium was discovered by H. Klaproth in year 1789 in Germany. Uranium was first isolated by E.-M. Péligot in 1841. Uranium derived its name from Uranus, the seventh planet in the Solar System.
| Discovered By | H. Klaproth |
| Discovery Date | 1789 in Germany |
| First Isolation | 1841 |
| Isolated by | E.-M. Péligot |
Klaproth mistakenly identified auranium oxideobtained frompitchblendeas the element itself and named it after the recently discovered planetUranus.
Uranium Uses
The element Uranium has Uranium is used as nuclear fuel for nuclear power reactors and produces the material needed for nuclear weapons. It is also used as a colorant for glass. It is also the major material from which other synthetic transuranium elements are made.. Since element Uranium has extremely short half-lives
Uranium Presence: Abundance in Nature and Around Us
The table below shows the abundance of Uranium in Universe, Sun, Meteorites, Earth's Crust, Oceans and Human Body.
| ppb by weight (1ppb =10^-7 %) | ppb by atoms (1ppb =10^-7 %) | |
|---|---|---|
| Abundance in Universe | 0.2 | 0.001 |
| Abundance in Sun | 1 | 0.004 |
| Abundance in Meteorites | 10 | 1 |
| Abundance in Earth's Crust | 1800 | 150 |
| Abundance in Oceans | 3.3 | 0.086 |
| Abundance in Humans | 1 | 0.03 |
Crystal Structure of Uranium
The solid state structure of Uranium is Base Centered Orthorhombic.
The Crystal structure can be described in terms of its unit Cell. The unit Cells repeats itself in three dimensional space to form the structure.
Unit Cell Parameters
The unit cell is represented in terms of its lattice parameters, which are the lengths of the cell edges Lattice Constants (a, b and c)
| a | b | c |
|---|---|---|
| 285.37 pm | 586.95 pm | 495.48 pm |
and the angles between them Lattice Angles (alpha, beta and gamma).
| alpha | beta | gamma |
|---|---|---|
| π/2 | π/2 | π/2 |
The positions of the atoms inside the unit cell are described by the set of atomic positions ( xi, yi, zi) measured from a reference lattice point.
The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space are described by the 230 space groups (219 distinct types, or 230 if chiral copies are considered distinct.
| Space Group Name | Cmcm |
| Space Group Number | 63 |
| Crystal Structure | Base Centered Orthorhombic |
| Number of atoms per unit cell | 2 |
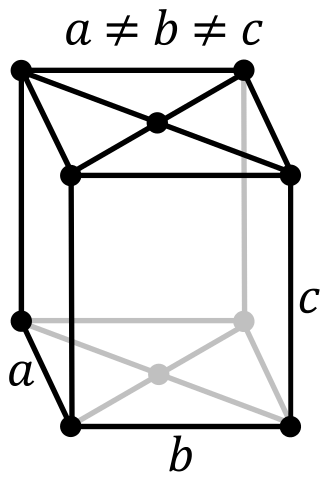
The number of atoms per unit cell in a simple cubic, face-centered cubic and body-centred cubic are 1,4,2 respectively.
Uranium Atomic and Orbital Properties
Uranium atoms have 92 electrons and the electronic shell structure is [2, 8, 18, 32, 21, 9, 2] with Atomic Term Symbol (Quantum Numbers) 5L6.
| Atomic Number | 92 |
| Number of Electrons (with no charge) | 92 |
| Number of Protons | 92 |
| Mass Number | 238 |
| Number of Neutrons | 146 |
| Shell structure (Electrons per energy level) | 2, 8, 18, 32, 21, 9, 2 |
| Electron Configuration | [Rn] 5f3 6d1 7s2 |
| Valence Electrons | 5f3 6d1 7s2 |
| Valence (Valency) | 6 |
| Main Oxidation States | 4, 6 |
| Oxidation States | -1, 1, 2, 3, 4, 5, 6 |
| Atomic Term Symbol (Quantum Numbers) | 5L6 |
Bohr Atomic Model of Uranium - Electrons per energy level
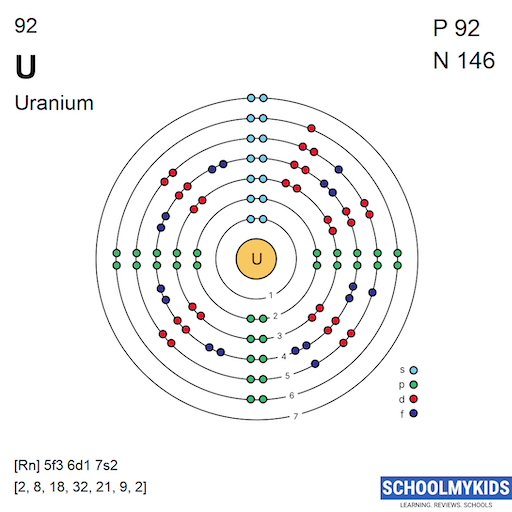
| n | s | p | d | f |
|---|
Ground State Electronic Configuration of Uranium - neutral Uranium atom
Abbreviated electronic configuration of Uranium
The ground state abbreviated electronic configuration of Neutral Uranium atom is [Rn] 5f3 6d1 7s2. The portion of Uranium configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Rn]. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used. This is important as it is the Valence electrons 5f3 6d1 7s2, electrons in the outermost shell that determine the chemical properties of the element.
Unabbreviated electronic configuration of neutral Uranium
Complete ground state electronic configuration for the Uranium atom, Unabbreviated electronic configuration
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f3 6d1 7s2
Electrons are filled in atomic orbitals as per the order determined by the Aufbau principle, Pauli Exclusion Principle and Hund’s Rule.
As per the Aufbau principle the electrons will occupy the orbitals having lower energies before occupying higher energy orbitals. According to this principle, electrons are filled in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p…
The Pauli exclusion principle states that a maximum of two electrons, each having opposite spins, can fit in an orbital.
Hund's rule states that every orbital in a given subshell is singly occupied by electrons before a second electron is filled in an orbital.
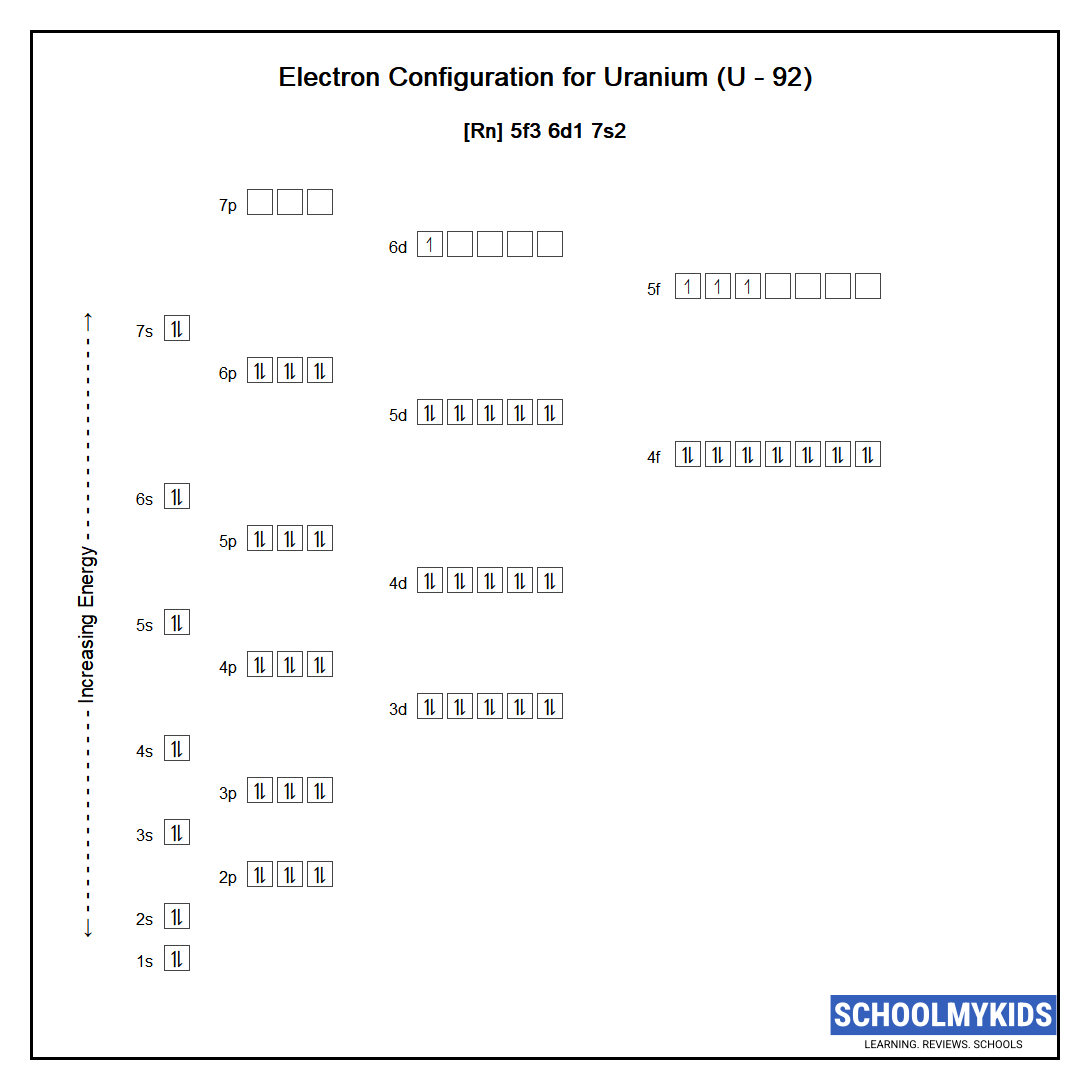
Atomic Structure of Uranium
Uranium atomic radius is 175 pm, while it's covalent radius is -.
| Atomic Radius Calculated | 175 pm(1.75 Å) |
| Atomic Radius Empirical | 175 pm (1.75 Å) |
| Atomic Volume | 12.495 cm3/mol |
| Covalent Radius | - |
| Van der Waals Radius | 186 pm |
| Neutron Cross Section | 7.57 |
| Neutron Mass Absorption | 0.0005 |
Spectral Lines of Uranium - Atomic Spectrum of Uranium
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from an excess or deficiency of photons in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules.
Spectral lines are the result of interaction between a quantum system and a single photon. A spectral line may be observed either as an emission line or an absorption line.
Spectral lines are highly atom-specific, and can be used to identify the chemical composition of any medium. Several elements, including helium, thallium, and caesium, were discovered by spectroscopic means. They are widely used to determine the physical conditions of stars and other celestial bodies that cannot be analyzed by other means.
Emission spectrum of Uranium

Absorption spectrum of Uranium

Uranium Chemical Properties: Uranium Ionization Energies and electron affinity
The electron affinity of Uranium is -.
| Valence | 6 |
| Electronegativity | 1.38 |
| ElectronAffinity | - |
Ionization Energy of Uranium
Ionization energy is the amount of energy required to remove an electron from an atom or molecule.in chemistry, this energy is expresed in kilocalories per mole (kcal/mol) or kilojoules per mole (kJ/mol).
Refer to table below for Ionization energies of Uranium
Here are the ionization energies of Uranium (U) both in electron volts (eV) and in kilojoules per mole (kJ/mol).
| Ionization energy number | Enthalpy in kJ/mol | Energy (eV) |
|---|---|---|
| 1st | 597.6 | 6.194 |
| 2nd | 1420 | 14.717 |
The conversion from kJ/mol to eV is done using the formula:
Energy (kJ/mol) = Energy (eV) x 96.485 Energy (kJ/mol)=Energy (eV)x96.485
where 1 eV = 96.485 kJ/mol.
1 electronvolt (eV) is equal to 96.485 kilojoules per mole (kJ/mol)
Uranium Physical Properties
Refer to below table for Uranium Physical Properties
| Density | 19.05 g/cm3(when liquid at m.p density is $17.3 g/cm3) |
| Molar Volume | 12.495 cm3/mol |
Elastic Properties
| Young Modulus | 208 |
| Shear Modulus | 111 GPa |
| Bulk Modulus | 100 GPa |
| Poisson Ratio | 0.23 |
Hardness of Uranium - Tests to Measure of Hardness of Element
| Mohs Hardness | 6 MPa |
| Vickers Hardness | 1960 MPa |
| Brinell Hardness | 2400 MPa |
Uranium Electrical Properties
Electrical resistivity measures element's electrical resistance or how strongly it resists electric current.The SI unit of electrical resistivity is the ohm-metre (Ω⋅m). While Electrical conductivity is the reciprocal of electrical resistivity. It represents a element's ability to conduct electric current. The SI unit of electrical conductivity is siemens per metre (S/m).
Uranium is a conductor of electricity. Refer to table below for the Electrical properties of Uranium
| Electrical conductors | Conductor |
| Electrical Conductivity | 3600000 S/m |
| Resistivity | 2.79e-7 m Ω |
| Superconducting Point | 0.68 |
Uranium Heat and Conduction Properties
| Thermal Conductivity | 27 W/(m K) |
| Thermal Expansion | 0.0000139 /K |
Uranium Magnetic Properties
| Magnetic Type | Paramagnetic |
| Curie Point | - |
| Mass Magnetic Susceptibility | 2.16e-8 m3/kg |
| Molar Magnetic Susceptibility | 5.14e-9 m3/mol |
| Volume Magnetic Susceptibility | 0.000411 |
Optical Properties of Uranium
| Refractive Index | - |
Acoustic Properties of Uranium
| Speed of Sound | 3155 m/s |
Uranium Thermal Properties - Enthalpies and thermodynamics
Refer to table below for Thermal properties of Uranium
| Melting Point | 1408 K(1134.85 °C, 2074.730 °F) |
| Boiling Point | 4200 K(3926.85 °C, 7100.330 °F) |
| Critical Temperature | - |
| Superconducting Point | 0.68 |
Enthalpies of Uranium
| Heat of Fusion | 14 kJ/mol |
| Heat of Vaporization | 420 kJ/mol |
| Heat of Combustion | - |
Uranium Isotopes - Nuclear Properties of Uranium
Uranium has 26 isotopes, with between 217 and 242 nucleons. Uranium has 0 stable naturally occuring isotopes.
Isotopes of Uranium - Naturally occurring stable Isotopes: -.
| Isotope | Z | N | Isotope Mass | % Abundance | T half | Decay Mode |
|---|---|---|---|---|---|---|
| 217U | 92 | 125 | 217 | Synthetic | ||
| 218U | 92 | 126 | 218 | Synthetic | ||
| 219U | 92 | 127 | 219 | Synthetic | ||
| 220U | 92 | 128 | 220 | Synthetic | ||
| 221U | 92 | 129 | 221 | Synthetic | ||
| 222U | 92 | 130 | 222 | Synthetic | ||
| 223U | 92 | 131 | 223 | Synthetic | ||
| 224U | 92 | 132 | 224 | Synthetic | ||
| 225U | 92 | 133 | 225 | Synthetic | ||
| 226U | 92 | 134 | 226 | Synthetic | ||
| 227U | 92 | 135 | 227 | Synthetic | ||
| 228U | 92 | 136 | 228 | Synthetic | ||
| 229U | 92 | 137 | 229 | Synthetic | ||
| 230U | 92 | 138 | 230 | Synthetic | ||
| 231U | 92 | 139 | 231 | Synthetic | ||
| 232U | 92 | 140 | 232 | Synthetic | ||
| 233U | 92 | 141 | 233 | Synthetic | ||
| 234U | 92 | 142 | 234 | 0.0055% | Stable | N/A |
| 235U | 92 | 143 | 235 | 0.72% | Stable | N/A |
| 236U | 92 | 144 | 236 | Synthetic | ||
| 237U | 92 | 145 | 237 | Synthetic | ||
| 238U | 92 | 146 | 238 | 99.2745% | 4.471×10^9 years | AlphaEmission |
| 239U | 92 | 147 | 239 | Synthetic | ||
| 240U | 92 | 148 | 240 | Synthetic | ||
| 241U | 92 | 149 | 241 | Synthetic | ||
| 242U | 92 | 150 | 242 | Synthetic |
Regulatory and Health - Health and Safety Parameters and Guidelines
The United States Department of Transportation (DOT) identifies hazard class of all dangerous elements/goods/commodities either by its class (or division) number or name. The DOT has divided these materials into nine different categories, known as Hazard Classes.
NFPA 704 is a Standard System for the Identification of the Hazards of Materials for Emergency Response. NFPA is a standard maintained by the US based National Fire Protection Association.
The health (blue), flammability (red), and reactivity (yellow) rating all use a numbering scale ranging from 0 to 4. A value of zero means that the element poses no hazard; a rating of four indicates extreme danger.
| NFPA Fire Rating | N/A | N/A |
| NFPA Health Rating | N/A | N/A |
| NFPA Reactivity Rating | N/A | N/A |
| NFPA Hazards | N/A |
| Autoignition Point | - |
| Flashpoint | - |
Database Search
List of unique identifiers to search the element in various chemical registry databases
| Database | Identifier number |
|---|---|
| CAS Number - Chemical Abstracts Service (CAS) | CAS7440-61-1 |
| RTECS Number | RTECSYR3490000 |
| CID Number | CID23989 |
| Gmelin Number | Gmelin16315 |
| NSC Number | - |
Compare Uranium with other elements
Compare Uranium with Group , Period 7 and Actinide elements of the periodic table.
Compare Uranium with all Group elements
Compare Uranium with all Period 7 elements
Compare Uranium with all Actinide elements
Frequently Asked Questions (FAQ)
Find the answers to the most frequently asked questions about Uranium