Neodymium (Nd) - Atomic, Physical & Chemical Properties, Uses, and Periodic Table Trends
Neodymium Element Information, Facts, Properties, Trends, Uses, Comparison with other elements
Neodymium (Nd) - Comprehensive Element Profile, Properties & Uses
In this comprehensive guide, you'll learn about Neodymium's unique chemical and physical properties, trends in the periodic table, isotopes, and its historical significance. We'll also cover its abundance, crystal structure, electron configuration, and health & safety guidelines. Explore how Neodymium compares with other elements and discover its many uses.
Neodymium is a chemical element with symbol Nd and atomic number 60. It is a soft silvery metal that tarnishes in air. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach.
Neodymium is part of Lanthanide series. You can also download Printable Periodic Table of Elements Flashcards for Neodymium in a PDF format.
Neodymium Facts
Read key information and facts about element Neodymium
| Name | Neodymium |
| Atomic Number | 60 |
| Atomic Symbol | Nd |
| Atomic Weight | 144.24 |
| Phase | Solid |
| Color | Silver |
| Appearance | silvery white |
| Classification | Lanthanide |
| Natural Occurance | Primordial |
| Group in Periodic Table | - |
| Group Name | |
| Period in Periodic Table | period 6 |
| Block in Periodic Table | f-block |
| Electronic Configuration | [Xe] 4f4 6s2 |
| Electronic Shell Structure (Electrons per shell) | 2, 8, 18, 22, 8, 2 |
| Melting Point | 1294 K |
| Boiling Point | 3373 K |
| CAS Number | CAS7440-00-8 |
How to Locate Neodymium on Periodic Table
Periodic table is arranged by atomic number, number of protons in the nucleus which is same as number of electrons. The atomic number increases from left to right. Periodic table starts at top left ( Atomic number 1) and ends at bottom right (atomic number 118). Therefore you can directly look for atomic number 60 to find Neodymium on periodic table.
Another way to read periodic table and locate an element is by using group number (column) and period number (row). To locate Neodymium on periodic table look for cross section of group - and period 6 in the modern periodic table.
Neodymium History
The element Neodymium was discovered by C. A. von Welsbach in year 1885 in Austria. Neodymium was first isolated by in . Neodymium derived its name from the Greek neos didymos meaning 'new twin'.
| Discovered By | C. A. von Welsbach |
| Discovery Date | 1885 in Austria |
| First Isolation | |
| Isolated by |
Von Welsbach discovered two new distinct elements in Mosander's didymia: praseodymium and neodymium.
Neodymium Uses
The element Neodymium has The major use for neodymium is in an alloy with iron and boron to make strong permanent magnets. Neodymium is used to make flint for lighters and is a component of specialized welders' goggles. Neodymium glass is used to make lasers, while Neodymium oxide and nitrate are used as catalysts in polymerization reactions.. Since element Neodymium has extremely short half-lives
Neodymium Presence: Abundance in Nature and Around Us
The table below shows the abundance of Neodymium in Universe, Sun, Meteorites, Earth's Crust, Oceans and Human Body.
| ppb by weight (1ppb =10^-7 %) | ppb by atoms (1ppb =10^-7 %) | |
|---|---|---|
| Abundance in Universe | 10 | 0.09 |
| Abundance in Sun | 3 | 0.02 |
| Abundance in Meteorites | 510 | 70 |
| Abundance in Earth's Crust | 33000 | 4800 |
| Abundance in Oceans | 0.0028 | 0.00012 |
| Abundance in Humans | - | - |
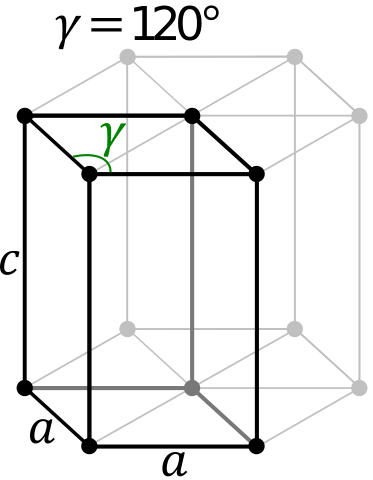
Crystal Structure of Neodymium
The solid state structure of Neodymium is Simple Hexagonal.
The Crystal structure can be described in terms of its unit Cell. The unit Cells repeats itself in three dimensional space to form the structure.
Unit Cell Parameters
The unit cell is represented in terms of its lattice parameters, which are the lengths of the cell edges Lattice Constants (a, b and c)
| a | b | c |
|---|---|---|
| 365.8 pm | 365.8 pm | 1179.9 pm |
and the angles between them Lattice Angles (alpha, beta and gamma).
| alpha | beta | gamma |
|---|---|---|
| π/2 | π/2 | 2 π/3 |
The positions of the atoms inside the unit cell are described by the set of atomic positions ( xi, yi, zi) measured from a reference lattice point.
The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space are described by the 230 space groups (219 distinct types, or 230 if chiral copies are considered distinct.
| Space Group Name | P63/mmc |
| Space Group Number | 194 |
| Crystal Structure | Simple Hexagonal |
| Number of atoms per unit cell |
The number of atoms per unit cell in a simple cubic, face-centered cubic and body-centred cubic are 1,4,2 respectively.
Neodymium Atomic and Orbital Properties
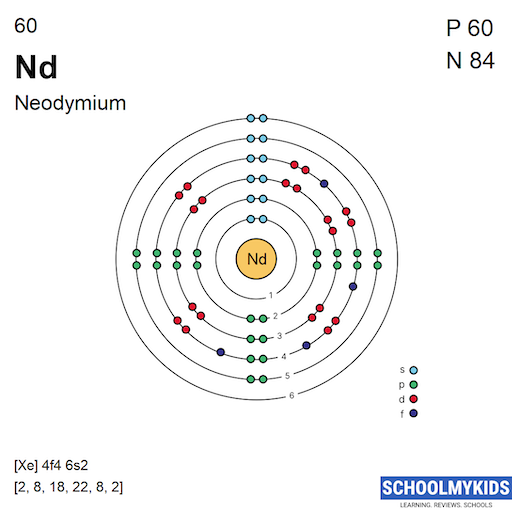
Neodymium atoms have 60 electrons and the electronic shell structure is [2, 8, 18, 22, 8, 2] with Atomic Term Symbol (Quantum Numbers) 5I4.
| Atomic Number | 60 |
| Number of Electrons (with no charge) | 60 |
| Number of Protons | 60 |
| Mass Number | 144 |
| Number of Neutrons | 84 |
| Shell structure (Electrons per energy level) | 2, 8, 18, 22, 8, 2 |
| Electron Configuration | [Xe] 4f4 6s2 |
| Valence Electrons | 4f4 6s2 |
| Valence (Valency) | 3 |
| Main Oxidation States | 3 |
| Oxidation States | 0, 2, 3, 4 |
| Atomic Term Symbol (Quantum Numbers) | 5I4 |
Bohr Atomic Model of Neodymium - Electrons per energy level
| n | s | p | d | f |
|---|
Ground State Electronic Configuration of Neodymium - neutral Neodymium atom
Abbreviated electronic configuration of Neodymium
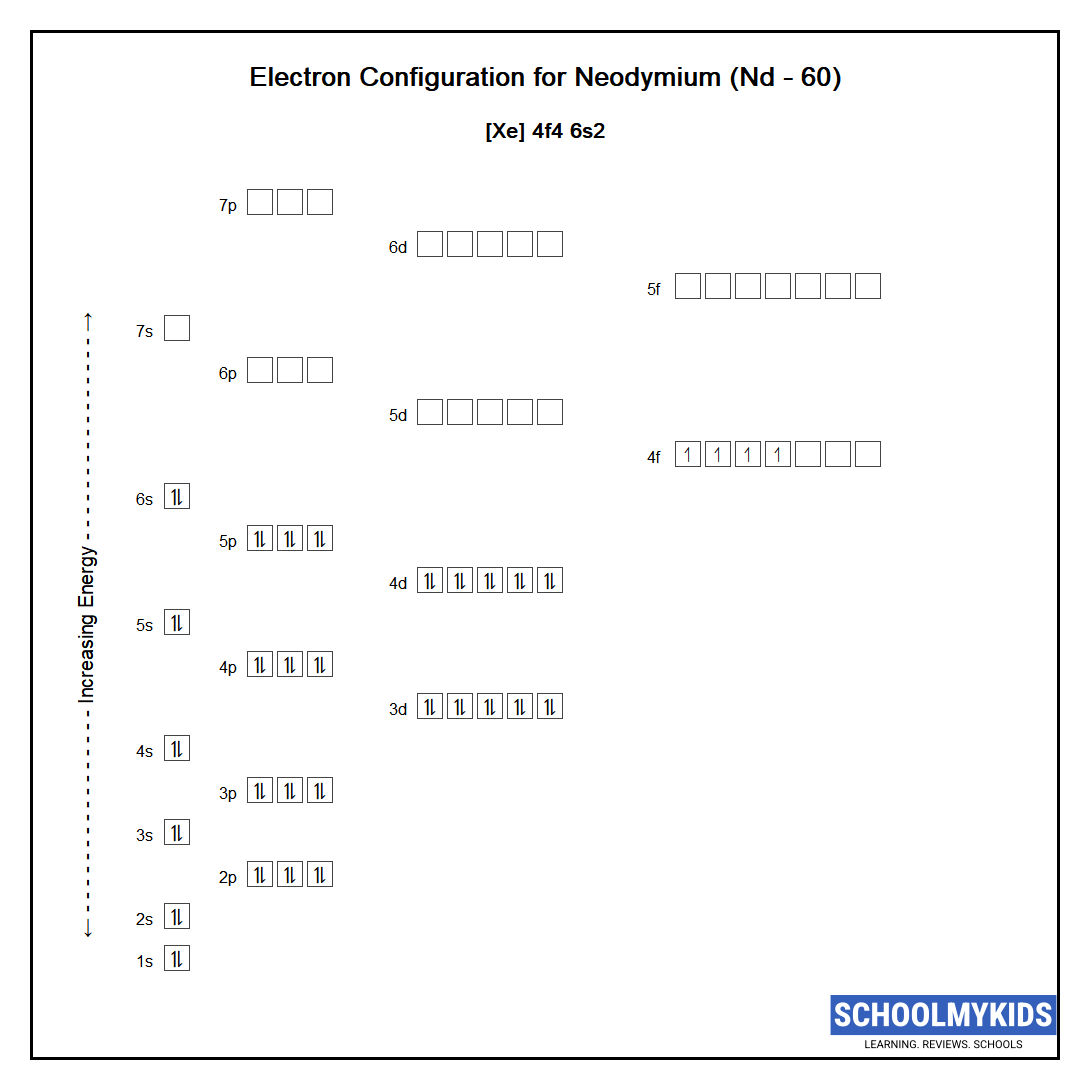
The ground state abbreviated electronic configuration of Neutral Neodymium atom is [Xe] 4f4 6s2. The portion of Neodymium configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Xe]. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used. This is important as it is the Valence electrons 4f4 6s2, electrons in the outermost shell that determine the chemical properties of the element.
Unabbreviated electronic configuration of neutral Neodymium
Complete ground state electronic configuration for the Neodymium atom, Unabbreviated electronic configuration
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f4 6s2
Electrons are filled in atomic orbitals as per the order determined by the Aufbau principle, Pauli Exclusion Principle and Hund’s Rule.
As per the Aufbau principle the electrons will occupy the orbitals having lower energies before occupying higher energy orbitals. According to this principle, electrons are filled in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p…
The Pauli exclusion principle states that a maximum of two electrons, each having opposite spins, can fit in an orbital.
Hund's rule states that every orbital in a given subshell is singly occupied by electrons before a second electron is filled in an orbital.
Atomic Structure of Neodymium
Neodymium atomic radius is 206 pm, while it's covalent radius is -.
| Atomic Radius Calculated | 206 pm(2.06 Å) |
| Atomic Radius Empirical | 185 pm (1.85 Å) |
| Atomic Volume | 20.576 cm3/mol |
| Covalent Radius | - |
| Van der Waals Radius | - |
| Neutron Cross Section | 49 |
| Neutron Mass Absorption | 0.011 |
Spectral Lines of Neodymium - Atomic Spectrum of Neodymium
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from an excess or deficiency of photons in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules.
Spectral lines are the result of interaction between a quantum system and a single photon. A spectral line may be observed either as an emission line or an absorption line.
Spectral lines are highly atom-specific, and can be used to identify the chemical composition of any medium. Several elements, including helium, thallium, and caesium, were discovered by spectroscopic means. They are widely used to determine the physical conditions of stars and other celestial bodies that cannot be analyzed by other means.
Emission spectrum of Neodymium

Absorption spectrum of Neodymium

Neodymium Chemical Properties: Neodymium Ionization Energies and electron affinity
The electron affinity of Neodymium is 50 kJ/mol.
| Valence | 3 |
| Electronegativity | 1.14 |
| ElectronAffinity | 50 kJ/mol |
Ionization Energy of Neodymium
Ionization energy is the amount of energy required to remove an electron from an atom or molecule.in chemistry, this energy is expresed in kilocalories per mole (kcal/mol) or kilojoules per mole (kJ/mol).
Refer to table below for Ionization energies of Neodymium
Here are the ionization energies of Neodymium (Nd) both in electron volts (eV) and in kilojoules per mole (kJ/mol).
| Ionization energy number | Enthalpy in kJ/mol | Energy (eV) |
|---|---|---|
| 1st | 533.1 | 5.525 |
| 2nd | 1040 | 10.779 |
| 3rd | 2130 | 22.076 |
| 4th | 3900 | 40.421 |
The conversion from kJ/mol to eV is done using the formula:
Energy (kJ/mol) = Energy (eV) x 96.485 Energy (kJ/mol)=Energy (eV)x96.485
where 1 eV = 96.485 kJ/mol.
1 electronvolt (eV) is equal to 96.485 kilojoules per mole (kJ/mol)
Neodymium Physical Properties
Refer to below table for Neodymium Physical Properties
| Density | 7.01 g/cm3(when liquid at m.p density is $6.89 g/cm3) |
| Molar Volume | 20.576 cm3/mol |
Elastic Properties
| Young Modulus | 41 |
| Shear Modulus | 16 GPa |
| Bulk Modulus | 32 GPa |
| Poisson Ratio | 0.28 |
Hardness of Neodymium - Tests to Measure of Hardness of Element
| Mohs Hardness | - |
| Vickers Hardness | 343 MPa |
| Brinell Hardness | 265 MPa |
Neodymium Electrical Properties
Electrical resistivity measures element's electrical resistance or how strongly it resists electric current.The SI unit of electrical resistivity is the ohm-metre (Ω⋅m). While Electrical conductivity is the reciprocal of electrical resistivity. It represents a element's ability to conduct electric current. The SI unit of electrical conductivity is siemens per metre (S/m).
Neodymium is a conductor of electricity. Refer to table below for the Electrical properties of Neodymium
| Electrical conductors | Conductor |
| Electrical Conductivity | 1600000 S/m |
| Resistivity | 6.4e-7 m Ω |
| Superconducting Point | - |
Neodymium Heat and Conduction Properties
| Thermal Conductivity | 17 W/(m K) |
| Thermal Expansion | 0.0000096 /K |
Neodymium Magnetic Properties
| Magnetic Type | Paramagnetic |
| Curie Point | - |
| Mass Magnetic Susceptibility | 4.8e-7 m3/kg |
| Molar Magnetic Susceptibility | 6.9235e-8 m3/mol |
| Volume Magnetic Susceptibility | 0.0033648 |
Optical Properties of Neodymium
| Refractive Index | - |
Acoustic Properties of Neodymium
| Speed of Sound | 2330 m/s |
Neodymium Thermal Properties - Enthalpies and thermodynamics
Refer to table below for Thermal properties of Neodymium
| Melting Point | 1294 K(1020.85 °C, 1869.530 °F) |
| Boiling Point | 3373 K(3099.85 °C, 5611.730 °F) |
| Critical Temperature | - |
| Superconducting Point | - |
Enthalpies of Neodymium
| Heat of Fusion | 7.1 kJ/mol |
| Heat of Vaporization | 285 kJ/mol |
| Heat of Combustion | - |
Neodymium Isotopes - Nuclear Properties of Neodymium
Neodymium has 38 isotopes, with between 124 and 161 nucleons. Neodymium has 5 stable naturally occuring isotopes.
Isotopes of Neodymium - Naturally occurring stable Isotopes: 142Nd, 143Nd, 145Nd, 146Nd, 148Nd.
| Isotope | Z | N | Isotope Mass | % Abundance | T half | Decay Mode |
|---|---|---|---|---|---|---|
| 124Nd | 60 | 64 | 124 | Synthetic | ||
| 125Nd | 60 | 65 | 125 | Synthetic | ||
| 126Nd | 60 | 66 | 126 | Synthetic | ||
| 127Nd | 60 | 67 | 127 | Synthetic | ||
| 128Nd | 60 | 68 | 128 | Synthetic | ||
| 129Nd | 60 | 69 | 129 | Synthetic | ||
| 130Nd | 60 | 70 | 130 | Synthetic | ||
| 131Nd | 60 | 71 | 131 | Synthetic | ||
| 132Nd | 60 | 72 | 132 | Synthetic | ||
| 133Nd | 60 | 73 | 133 | Synthetic | ||
| 134Nd | 60 | 74 | 134 | Synthetic | ||
| 135Nd | 60 | 75 | 135 | Synthetic | ||
| 136Nd | 60 | 76 | 136 | Synthetic | ||
| 137Nd | 60 | 77 | 137 | Synthetic | ||
| 138Nd | 60 | 78 | 138 | Synthetic | ||
| 139Nd | 60 | 79 | 139 | Synthetic | ||
| 140Nd | 60 | 80 | 140 | Synthetic | ||
| 141Nd | 60 | 81 | 141 | Synthetic | ||
| 142Nd | 60 | 82 | 142 | 27.2% | Stable | N/A |
| 143Nd | 60 | 83 | 143 | 12.2% | Stable | N/A |
| 144Nd | 60 | 84 | 144 | 23.8% | Stable | |
| 145Nd | 60 | 85 | 145 | 8.3% | Stable | N/A |
| 146Nd | 60 | 86 | 146 | 17.2% | Stable | N/A |
| 147Nd | 60 | 87 | 147 | Synthetic | ||
| 148Nd | 60 | 88 | 148 | 5.7% | Stable | N/A |
| 149Nd | 60 | 89 | 149 | Synthetic | ||
| 150Nd | 60 | 90 | 150 | 5.6% | Stable | N/A |
| 151Nd | 60 | 91 | 151 | Synthetic | ||
| 152Nd | 60 | 92 | 152 | Synthetic | ||
| 153Nd | 60 | 93 | 153 | Synthetic | ||
| 154Nd | 60 | 94 | 154 | Synthetic | ||
| 155Nd | 60 | 95 | 155 | Synthetic | ||
| 156Nd | 60 | 96 | 156 | Synthetic | ||
| 157Nd | 60 | 97 | 157 | Synthetic | ||
| 158Nd | 60 | 98 | 158 | Synthetic | ||
| 159Nd | 60 | 99 | 159 | Synthetic | ||
| 160Nd | 60 | 100 | 160 | Synthetic | ||
| 161Nd | 60 | 101 | 161 | Synthetic |
Regulatory and Health - Health and Safety Parameters and Guidelines
The United States Department of Transportation (DOT) identifies hazard class of all dangerous elements/goods/commodities either by its class (or division) number or name. The DOT has divided these materials into nine different categories, known as Hazard Classes.
NFPA 704 is a Standard System for the Identification of the Hazards of Materials for Emergency Response. NFPA is a standard maintained by the US based National Fire Protection Association.
The health (blue), flammability (red), and reactivity (yellow) rating all use a numbering scale ranging from 0 to 4. A value of zero means that the element poses no hazard; a rating of four indicates extreme danger.
| NFPA Fire Rating | N/A | N/A |
| NFPA Health Rating | N/A | N/A |
| NFPA Reactivity Rating | N/A | N/A |
| NFPA Hazards | N/A |
| Autoignition Point | - |
| Flashpoint | - |
Database Search
List of unique identifiers to search the element in various chemical registry databases
| Database | Identifier number |
|---|---|
| CAS Number - Chemical Abstracts Service (CAS) | CAS7440-00-8 |
| RTECS Number | RTECSQO8575000 |
| CID Number | CID23934 |
| Gmelin Number | - |
| NSC Number | - |
Compare Neodymium with other elements
Compare Neodymium with Group , Period 6 and Lanthanide elements of the periodic table.
Compare Neodymium with all Group elements
Compare Neodymium with all Period 6 elements
Compare Neodymium with all Lanthanide elements
Frequently Asked Questions (FAQ)
Find the answers to the most frequently asked questions about Neodymium