Compare Uranium vs Helium: Periodic Table Element Comparison Table and Properties
Compare the elements Uranium and Helium on the basis of their properties, attributes and periodic table facts. Compare elements - Uranium and Helium comparison table side by side across over 90 properties. All the elements of similar categories show a lot of similarities and differences in their chemical, atomic, physical properties and uses. These similarities and dissimilarities should be known while we study periodic table elements. You can study the detailed comparison between Uranium vs Helium with most reliable information about their properties, attributes, facts, uses etc. You can compare U vs He on more than 90 properties like electronegativity, oxidation state, atomic shells, orbital structure, Electronaffinity, physical states, electrical conductivity and many more. This in-depth comparison helps students, educators, researchers, and science enthusiasts understand the differences and similarities between Uranium and Helium.
Uranium and Helium Comparison
Here's a detailed comparison between Uranium (U) and Helium (He), focusing on their position in the periodic table, physical and chemical properties, stability, and uses.
Facts - Basic Element Details
| Name | Uranium | Helium |
|---|---|---|
| Atomic Number | 92 | 2 |
| Atomic Symbol | U | He |
| Atomic Weight | 238.02891 | 4.002602 |
| Phase at STP | Solid | Gas |
| Color | Silver | Colorless |
| Metallic Classification | Actinide | Noble Gas |
| Group in Periodic Table | Actinide (no group number) | group 18 |
| Group Name | helium family or neon family | |
| Period in Periodic Table | period 7 | period 1 |
| Block in Periodic Table | f -block | p -block |
| Electronic Configuration | [Rn] 5f3 6d1 7s2 | 1s2 |
| Electronic Shell Structure (Electrons per shell) | 2, 8, 18, 32, 21, 9, 2 | 2 |
| Melting Point | 1408 K | 0 K |
| Boiling Point | 4200 K | 4.22 K |
| CAS Number | CAS7440-61-1 | CAS7440-59-7 |
| Neighborhood Elements | Neighborhood Elements of Uranium | Neighborhood Elements of Helium |
History
| Parameter | Uranium | Helium |
|---|---|---|
| History | The element Uranium was discovered by H. Klaproth in year 1789 in Germany. Uranium derived its name from Uranus, the seventh planet in the Solar System. | The element Helium was discovered by P. Janssen and N. Lockyer in year 1868 in Sweden and United Kingdom. Helium derived its name from the Greek word helios, meaning 'sun'. |
| Discovery | H. Klaproth (1789) | P. Janssen and N. Lockyer (1868) |
| Isolated | E.-M. Péligot (1841) | W. Ramsay,T. Cleve, and N. Langlet (1895) |
Presence: Abundance in Nature and Around Us
Parts per billion (ppb) by weight / by atoms (1ppb =10^-7 %)
| Property | Uranium | Helium |
|---|---|---|
| Abundance in Universe | 0.2 / 0.001 | 230000000 / 72000000 |
| Abundance in Sun | 1 / 0.004 | 230000000 / 74000000 |
| Abundance in Meteorites | 10 / 1 | - / - |
| Abundance in Earth's Crust | 1800 / 150 | 5.5 / 30 |
| Abundance in Oceans | 3.3 / 0.086 | 0.0072 / 0.011 |
| Abundance in Humans | 1 / 0.03 | - / - |
Crystal Structure and Atomic Structure
| Property | Uranium | Helium |
|---|---|---|
| Atomic Volume | 12.495 cm3/mol | 22.4136 cm3/mol |
| Atomic Radius | 175 pm | 31 pm |
| Covalent Radius | - | 32 pm |
| Van der Waals Radius | 186 pm | 140 pm |
Atomic Spectrum - Spectral Lines | ||
| Emission Spectrum |  |  |
| Absorption Spectrum |  |  |
| Lattice Constant | 285.37, 586.95, 495.48 pm | 424.2, 424.2, 424.2 pm |
| Lattice Angle | π/2, π/2, π/2 | π/2, π/2, π/2 |
| Space Group Name | Cmcm | Fm_ 3m |
| Space Group Number | 63 | 225 |
| Crystal Structure | Base Centered Orthorhombic 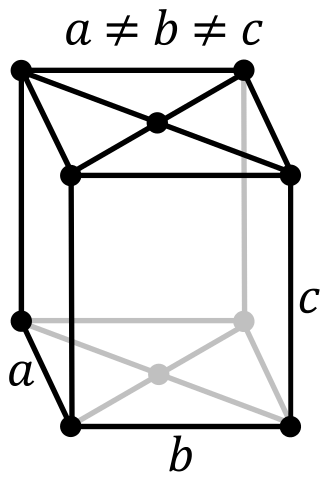 | Face Centered Cubic 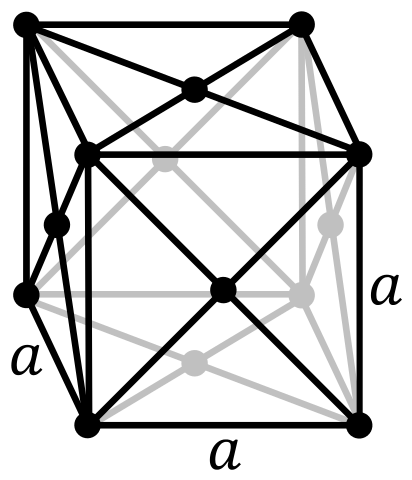 |
Atomic and Orbital Properties
| Property | Uranium | Helium |
|---|---|---|
| Atomic Number | 92 | 2 |
| Number of Electrons (with no charge) | 92 | 2 |
| Number of Protons | 92 | 2 |
| Mass Number | 238.02891 | 4.002602 |
| Number of Neutrons | 146 | 2 |
| Shell structure (Electrons per energy level) | 2, 8, 18, 32, 21, 9, 2 | 2 |
| Electron Configuration | [Rn] 5f3 6d1 7s2 | 1s2 |
| Valence Electrons | 5f3 6d1 7s2 | 1s2 |
| Oxidation State | 4, 6 | - |
| Atomic Term Symbol (Quantum Numbers) | 5L6 | 1S0 |
| Shell structure | 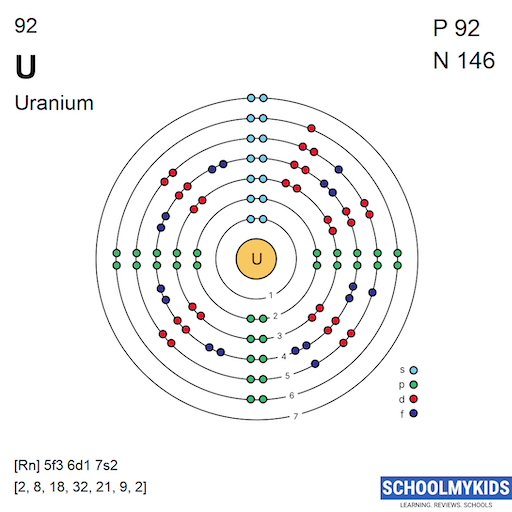 | 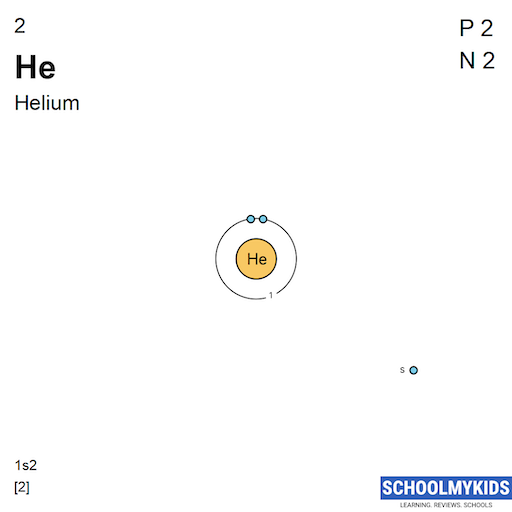 |
Isotopes and Nuclear Properties
Uranium has 0 stable naturally occuring isotopes while Helium has 2 stable naturally occuring isotopes.
| Parameter | Uranium | Helium |
|---|---|---|
| Known Isotopes | 217U, 218U, 219U, 220U, 221U, 222U, 223U, 224U, 225U, 226U, 227U, 228U, 229U, 230U, 231U, 232U, 233U, 234U, 235U, 236U, 237U, 238U, 239U, 240U, 241U, 242U | 3He, 4He, 5He, 6He, 7He, 8He, 9He, 10He |
| Stable Isotopes | - | Naturally occurring stable isotopes: 3He, 4He |
| Neutron Cross Section | 7.57 | 0.007 |
| Neutron Mass Absorption | 0.0005 | 0.00001 |
Chemical Properties: Ionization Energies and electron affinity
| Property | Uranium | Helium |
|---|---|---|
| Valence or Valency | 6 | 0 |
| Electronegativity | 1.38 Pauling Scale | - |
| Oxidation State | 4, 6 | - |
| Electron Affinity | - | 0 kJ/mol |
| Ionization Energies | 1st: 597.6 kJ/mol 2nd: 1420 kJ/mol | 1st: 2372.3 kJ/mol 2nd: 5250.5 kJ/mol |
Physical Properties
Helium (0.0001785 g/cm³) is less dense than Uranium (19.05 g/cm³). This means that a given volume of Uranium will be heavier than the same volume of Helium. Uranium is about 10672168.9 denser than Helium
| Property | Uranium | Helium |
|---|---|---|
| Phase at STP | Solid | Gas |
| Color | Silver | Colorless |
| Density | 19.05 g/cm3 | 0.0001785 g/cm3 |
| Density (when liquid (at melting point)) | 17.3 g/cm3 | - |
| Molar Volume | 12.495 cm3/mol | 22.4136 cm3/mol |
Mechanical and Hardness Properties
| Property | Uranium | Helium |
|---|---|---|
Elastic Properties | ||
| Young Modulus | 208 | - |
| Shear Modulus | 111 GPa | - |
| Bulk Modulus | 100 GPa | - |
| Poisson Ratio | 0.23 | - |
Hardness - Tests to Measure of Hardness of Element | ||
| Mohs Hardness | 6 MPa | - |
| Vickers Hardness | 1960 MPa | - |
| Brinell Hardness | 2400 MPa | - |
Thermal and Electrical Conductivity
| Property | Uranium | Helium |
|---|---|---|
Heat and Conduction Properties | ||
| Thermal Conductivity | 27 W/(m K) | 0.1513 W/(m K) |
| Thermal Expansion | 0.0000139 /K | - |
Electrical Properties | ||
| Electrical Conductivity | 3600000 S/m | - |
| Resistivity | 2.79e-7 m Ω | - |
| Superconducting Point | 0.68 | - |
Magnetic and Optical Properties
| Property | Uranium | Helium |
|---|---|---|
Magnetic Properties | ||
| Magnetic Type | Paramagnetic | Diamagnetic |
| Curie Point | - | - |
| Mass Magnetic Susceptibility | 2.16e-8 m3/kg | -5.9e-9 m3/kg |
| Molar Magnetic Susceptibility | 5.14e-9 m3/mol | -2.36e-11 m3/mol |
| Volume Magnetic Susceptibility | 0.000411 | -1.05e-9 |
Optical Properties | ||
| Refractive Index | - | 1.000035 |
Acoustic Properties | ||
| Speed of Sound | 3155 m/s | 970 m/s |
Thermal Properties - Enthalpies and thermodynamics
| Property | Uranium | Helium |
|---|---|---|
| Melting Point | 1408 K | 0 K |
| Boiling Point | 4200 K | 4.22 K |
| Critical Temperature | - | 5.19 K |
| Superconducting Point | 0.68 | - |
Enthalpies | ||
| Heat of Fusion | 14 kJ/mol | 0.02 kJ/mol |
| Heat of Vaporization | 420 kJ/mol | 0.083 kJ/mol |
| Heat of Combustion | - | - |
Regulatory and Health - Health and Safety Parameters and Guidelines
| Parameter | Uranium | Helium |
|---|---|---|
| CAS Number | CAS7440-61-1 | CAS7440-59-7 |
| RTECS Number | RTECSYR3490000 | RTECSMH6520000 |
| DOT Hazard Class | 7 | 2.2 |
| DOT Numbers | 2979 | 1963 |
| EU Number | EU231-170-6 | - |
| NFPA Fire Rating | - | 0 |
| NFPA Health Rating | - | 1 |
| NFPA Reactivity Rating | - | 0 |
| NFPA Hazards | - | - |
| AutoIgnition Point | - | - |
| Flashpoint | - | - |
Compare Uranium and Helium With Other Elements
Compare Uranium and Helium with other elements of the periodic table. Explore howUranium and Helium stack up against other elements of the periodic table. Use our interactive comparison tool to analyze 90+ properties across different metals, non-metals, metalloids, and noble gases. Understanding these differences is crucial for applications in engineering, chemistry, electronics, biology, and material science.

