Pa Protactinium
Element Information, Facts, Properties, Trends, Uses, Comparison with other elements
Protactinium is a chemical element with symbol Pa and atomic number 91. It is a dense, silvery-gray metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds where protactinium is usually present in the oxidation state +5, but can also assume +4 and even +2 or +3 states.
It belongs to group null of the periodic table having trivial name . You can also download Printable Periodic Table of Elements Flashcards for Protactinium in a PDF format.
Protactinium Facts
Read key information and facts about element Protactinium
| Name | Protactinium |
| Atomic Number | 91 |
| Atomic Symbol | Pa |
| Atomic Weight | 231.03588 |
| Phase | Solid |
| Color | Silver |
| Appearance | bright, silvery metallic luster |
| Classification | Actinide |
| Natural Occurance | From decay |
| Group in Periodic Table | - |
| Group Name | |
| Period in Periodic Table | period 7 |
| Block in Periodic Table | f-block |
| Electronic Configuration | [Rn] 5f2 6d1 7s2 |
| Electronic Shell Structure (Electrons per shell) | 2, 8, 18, 32, 20, 9, 2 |
| Melting Point | 1845 K |
| Boiling Point | 4273 K |
| CAS Number | CAS7440-13-3 |
How to Locate Protactinium on Periodic Table
Periodic table is arranged by atomic number, number of protons in the nucleus which is same as number of electrons. The atomic number increases from left to right. Periodic table starts at top left ( Atomic number 1) and ends at bottom right (atomic number 118). Therefore you can directly look for atomic number 91 to find Protactinium on periodic table.
Another way to read periodic table and locate an element is by using group number (column) and period number (row). To locate Protactinium on periodic table look for cross section of group - and period 7 in the modern periodic table.
Protactinium History
The element Protactinium was discovered by O. H. Göhring and K. Fajans in year 1913 in Germany and United Kingdom. Protactinium was first isolated by A. von Grosse in 1927. Protactinium derived its name from the Greek protos, 'first', and actinium, which is produced through the radioactive decay of protactinium.
| Discovered By | O. H. Göhring and K. Fajans |
| Discovery Date | 1913 in Germany and United Kingdom |
| First Isolation | 1927 |
| Isolated by | A. von Grosse |
The two obtained the first isotope of this element that had been predicted by Mendeleev in 1871 as a member of the natural decay of 238U. Originally isolated in 1900 by William Crookes, who nevertheless did not recognize that it was a new element.
Protactinium Uses
There are currently no commercial uses for protactinium due to its relative rarity.
Protactinium Presence: Abundance in Nature and Around Us
The table below shows the abundance of Protactinium in Universe, Sun, Meteorites, Earth's Crust, Oceans and Human Body.
| ppb by weight (1ppb =10^-7 %) | ppb by atoms (1ppb =10^-7 %) | |
|---|---|---|
| Abundance in Universe | - | - |
| Abundance in Sun | - | - |
| Abundance in Meteorites | - | - |
| Abundance in Earth's Crust | 0.000010 | 0.0000009 |
| Abundance in Oceans | 0.0000000000000002 | 0.000000000000000005 |
| Abundance in Humans | - | - |
Crystal Structure of Protactinium
The solid state structure of Protactinium is Centered Tetragonal.
The Crystal structure can be described in terms of its unit Cell. The unit Cells repeats itself in three dimensional space to form the structure.
Unit Cell Parameters
The unit cell is represented in terms of its lattice parameters, which are the lengths of the cell edges Lattice Constants (a, b and c)
| a | b | c |
|---|---|---|
| 392.5 pm | 392.5 pm | 323.8 pm |
and the angles between them Lattice Angles (alpha, beta and gamma).
| alpha | beta | gamma |
|---|---|---|
| π/2 | π/2 | π/2 |
The positions of the atoms inside the unit cell are described by the set of atomic positions ( xi, yi, zi) measured from a reference lattice point.
The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space are described by the 230 space groups (219 distinct types, or 230 if chiral copies are considered distinct.
| Space Group Name | I4/mmm |
| Space Group Number | 139 |
| Crystal Structure | Centered Tetragonal |
| Number of atoms per unit cell |
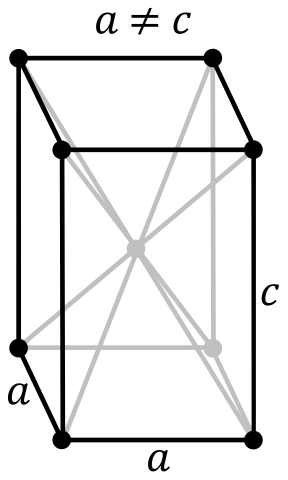
The number of atoms per unit cell in a simple cubic, face-centered cubic and body-centred cubic are 1,4,2 respectively.
Protactinium Atomic and Orbital Properties
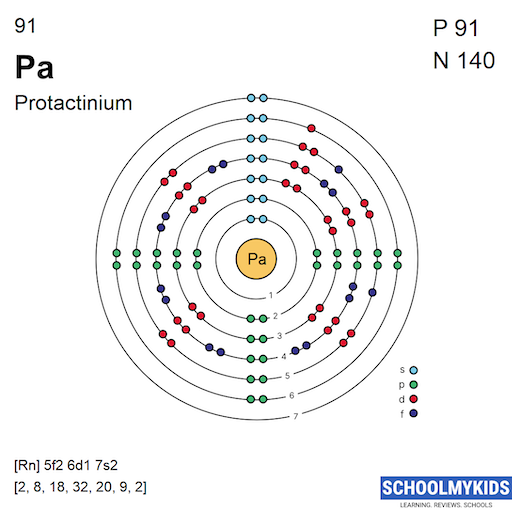
Protactinium atoms have 91 electrons and the electronic shell structure is [2, 8, 18, 32, 20, 9, 2] with Atomic Term Symbol (Quantum Numbers) 4K11/2.
| Atomic Number | 91 |
| Number of Electrons (with no charge) | 91 |
| Number of Protons | 91 |
| Mass Number | 231 |
| Number of Neutrons | 140 |
| Shell structure (Electrons per energy level) | 2, 8, 18, 32, 20, 9, 2 |
| Electron Configuration | [Rn] 5f2 6d1 7s2 |
| Valence Electrons | 5f2 6d1 7s2 |
| Valence (Valency) | 5 |
| Main Oxidation States | 5 |
| Oxidation States | 2, 3, 4, 5 |
| Atomic Term Symbol (Quantum Numbers) | 4K11/2 |
Bohr Atomic Model of Protactinium - Electrons per energy level
| n | s | p | d | f |
|---|
Ground State Electronic Configuration of Protactinium - neutral Protactinium atom
Abbreviated electronic configuration of Protactinium
The ground state abbreviated electronic configuration of Neutral Protactinium atom is [Rn] 5f2 6d1 7s2. The portion of Protactinium configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Rn]. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used. This is important as it is the Valence electrons 5f2 6d1 7s2, electrons in the outermost shell that determine the chemical properties of the element.
Unabbreviated electronic configuration of neutral Protactinium
Complete ground state electronic configuration for the Protactinium atom, Unabbreviated electronic configuration
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f2 6d1 7s2
Electrons are filled in atomic orbitals as per the order determined by the Aufbau principle, Pauli Exclusion Principle and Hund’s Rule.
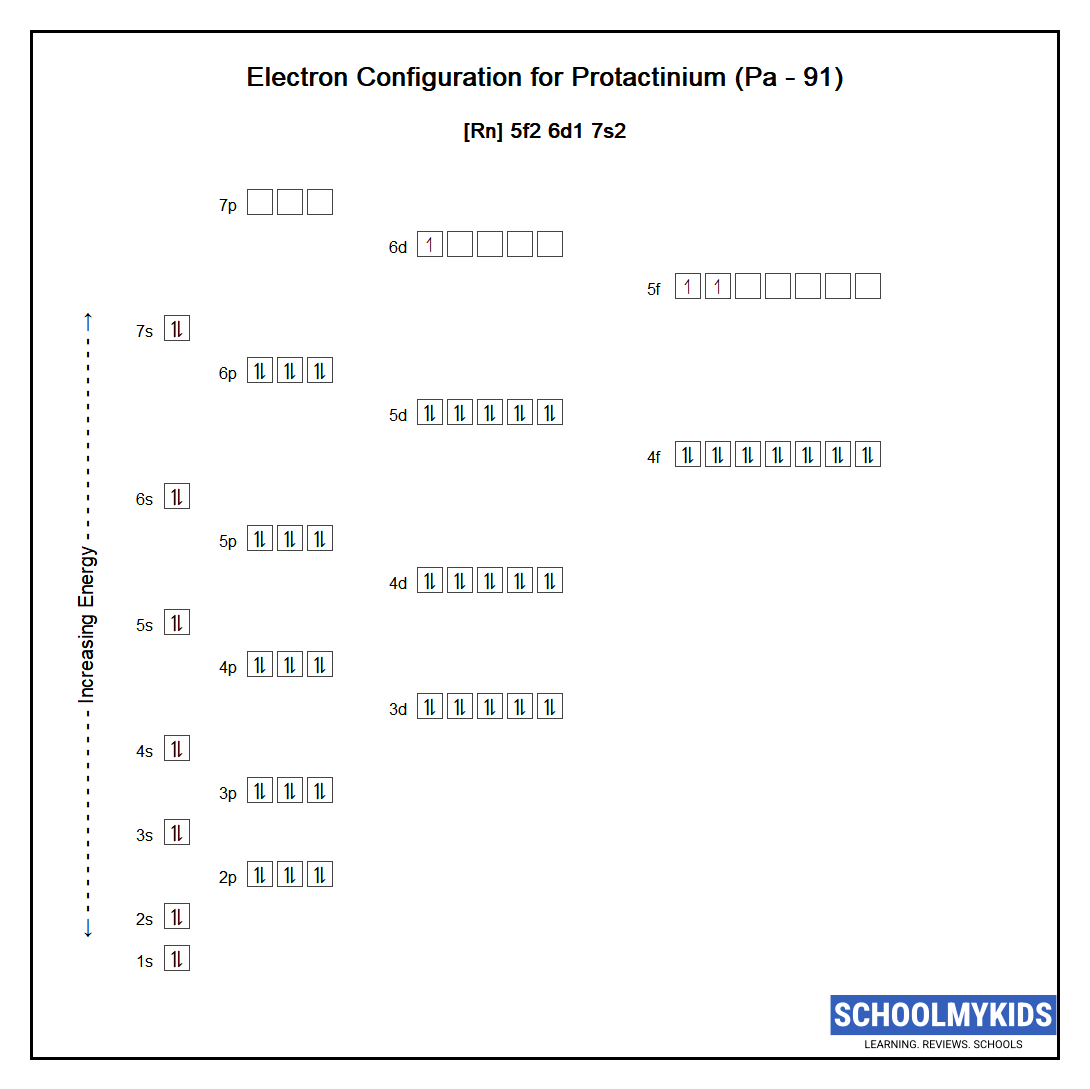
Atomic Structure of Protactinium
Protactinium atomic radius is 180 pm, while it's covalent radius is -.
| Atomic Radius Calculated | 180 pm(1.8 Å) |
| Atomic Radius Empirical | 180 pm (1.8 Å) |
| Atomic Volume | 15.18 cm3/mol |
| Covalent Radius | - |
| Van der Waals Radius | - |
| Neutron Cross Section | 500 |
| Neutron Mass Absorption | - |
Spectral Lines of Protactinium - Atomic Spectrum of Protactinium
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from an excess or deficiency of photons in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules.
Spectral lines are the result of interaction between a quantum system and a single photon. A spectral line may be observed either as an emission line or an absorption line.
Spectral lines are highly atom-specific, and can be used to identify the chemical composition of any medium. Several elements, including helium, thallium, and caesium, were discovered by spectroscopic means. They are widely used to determine the physical conditions of stars and other celestial bodies that cannot be analyzed by other means.
Emission spectrum of Protactinium

Absorption spectrum of Protactinium

Protactinium Chemical Properties: Protactinium Ionization Energies and electron affinity
The electron affinity of Protactinium is -.
| Valence | 5 |
| Electronegativity | 1.5 |
| ElectronAffinity | - |
Ionization Energy of Protactinium
Ionization energy is the amount of energy required to remove an electron from an atom or molecule.in chemistry, this energy is expresed in kilocalories per mole (kcal/mol) or kilojoules per mole (kJ/mol).
Refer to table below for Ionization energies of Protactinium
| Ionization energy number | Enthalpy - kJ/mol |
|---|---|
| 1st | 568 |
Protactinium Physical Properties
Refer to below table for Protactinium Physical Properties
| Density | 15.37 g/cm3 |
| Molar Volume | 15.18 cm3/mol |
Elastic Properties
| Young Modulus | - |
| Shear Modulus | - |
| Bulk Modulus | - |
| Poisson Ratio | - |
Hardness of Protactinium - Tests to Measure of Hardness of Element
| Mohs Hardness | - |
| Vickers Hardness | - |
| Brinell Hardness | - |
Protactinium Electrical Properties
Electrical resistivity measures element's electrical resistance or how strongly it resists electric current.The SI unit of electrical resistivity is the ohm-metre (Ω⋅m). While Electrical conductivity is the reciprocal of electrical resistivity. It represents a element's ability to conduct electric current. The SI unit of electrical conductivity is siemens per metre (S/m).
Protactinium is a conductor of electricity. Refer to table below for the Electrical properties of Protactinium
| Electrical conductors | Conductor |
| Electrical Conductivity | 5600000 S/m |
| Resistivity | 1.8e-7 m Ω |
| Superconducting Point | 1.4 |
Protactinium Heat and Conduction Properties
| Thermal Conductivity | 47 W/(m K) |
| Thermal Expansion | - |
Protactinium Magnetic Properties
| Magnetic Type | Paramagnetic |
| Curie Point | - |
| Mass Magnetic Susceptibility | 3.25e-8 m3/kg |
| Molar Magnetic Susceptibility | 7.509e-9 m3/mol |
| Volume Magnetic Susceptibility | 0.0004995 |
Optical Properties of Protactinium
| Refractive Index | - |
Acoustic Properties of Protactinium
| Speed of Sound | - |
Protactinium Thermal Properties - Enthalpies and thermodynamics
Refer to table below for Thermal properties of Protactinium
| Melting Point | 1845 K(1571.85 °C, 2861.330 °F) |
| Boiling Point | 4273 K(3999.85 °C, 7231.730 °F) |
| Critical Temperature | - |
| Superconducting Point | 1.4 |
Enthalpies of Protactinium
| Heat of Fusion | 15 kJ/mol |
| Heat of Vaporization | 470 kJ/mol |
| Heat of Combustion | - |
Protactinium Isotopes - Nuclear Properties of Protactinium
Protactinium has 29 isotopes, with between 212 and 240 nucleons. Protactinium has 0 stable naturally occuring isotopes.
Isotopes of Protactinium - Naturally occurring stable Isotopes: -.
| Isotope | Z | N | Isotope Mass | % Abundance | T half | Decay Mode |
|---|---|---|---|---|---|---|
| 212Pa | 91 | 121 | 212 | Synthetic | ||
| 213Pa | 91 | 122 | 213 | Synthetic | ||
| 214Pa | 91 | 123 | 214 | Synthetic | ||
| 215Pa | 91 | 124 | 215 | Synthetic | ||
| 216Pa | 91 | 125 | 216 | Synthetic | ||
| 217Pa | 91 | 126 | 217 | Synthetic | ||
| 218Pa | 91 | 127 | 218 | Synthetic | ||
| 219Pa | 91 | 128 | 219 | Synthetic | ||
| 220Pa | 91 | 129 | 220 | Synthetic | ||
| 221Pa | 91 | 130 | 221 | Synthetic | ||
| 222Pa | 91 | 131 | 222 | Synthetic | ||
| 223Pa | 91 | 132 | 223 | Synthetic | ||
| 224Pa | 91 | 133 | 224 | Synthetic | ||
| 225Pa | 91 | 134 | 225 | Synthetic | ||
| 226Pa | 91 | 135 | 226 | Synthetic | ||
| 227Pa | 91 | 136 | 227 | Synthetic | ||
| 228Pa | 91 | 137 | 228 | Synthetic | ||
| 229Pa | 91 | 138 | 229 | Synthetic | ||
| 230Pa | 91 | 139 | 230 | Synthetic | ||
| 231Pa | 91 | 140 | 231 | Synthetic | 32788 years | AlphaEmission |
| 232Pa | 91 | 141 | 232 | Synthetic | ||
| 233Pa | 91 | 142 | 233 | Synthetic | ||
| 234Pa | 91 | 143 | 234 | Synthetic | ||
| 235Pa | 91 | 144 | 235 | Synthetic | ||
| 236Pa | 91 | 145 | 236 | Synthetic | ||
| 237Pa | 91 | 146 | 237 | Synthetic | ||
| 238Pa | 91 | 147 | 238 | Synthetic | ||
| 239Pa | 91 | 148 | 239 | Synthetic | ||
| 240Pa | 91 | 149 | 240 | Synthetic |
Regulatory and Health - Health and Safety Parameters and Guidelines
The United States Department of Transportation (DOT) identifies hazard class of all dangerous elements/goods/commodities either by its class (or division) number or name. The DOT has divided these materials into nine different categories, known as Hazard Classes.
NFPA 704 is a Standard System for the Identification of the Hazards of Materials for Emergency Response. NFPA is a standard maintained by the US based National Fire Protection Association.
The health (blue), flammability (red), and reactivity (yellow) rating all use a numbering scale ranging from 0 to 4. A value of zero means that the element poses no hazard; a rating of four indicates extreme danger.
| NFPA Fire Rating | N/A | N/A |
| NFPA Health Rating | N/A | N/A |
| NFPA Reactivity Rating | N/A | N/A |
| NFPA Hazards | N/A |
| Autoignition Point | - |
| Flashpoint | - |
Database Search
List of unique identifiers to search the element in various chemical registry databases
| Database | Identifier number |
|---|---|
| CAS Number - Chemical Abstracts Service (CAS) | CAS7440-13-3 |
| RTECS Number | - |
| CID Number | - |
| Gmelin Number | - |
| NSC Number | - |
Compare Protactinium with other elements
Compare Protactinium with all Group elements
Compare Protactinium with all Period 7 elements
Compare Protactinium with all Actinide elements
FAQs
What is the electronic configuration of Protactinium?
The electronic configuration of Protactinium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f2 6d1 7s2.
What is the abbreviated electronic configuration of Protactinium?
The abbreviated electronic configuration of Protactinium is [Rn] 5f2 6d1 7s2. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the preceding period in square brackets.
What is the symbol of Protactinium?
Symbol of Protactinium is Pa. Protactinium is a chemical element with symbol Pa and atomic number 91.
What is the position of Protactinium in the Periodic Table?
Protactinium is a chemical element with the symbol Pa and atomic number 91. Protactinium is the 91 element on the periodic table. It is located in group null and period 7 in the modern periodic table.
What is the atomic number of Protactinium?
The atomic number of Protactinium is 91.
What is the color of Protactinium?
Protactinium is of Silver color.
Who discovered Protactinium?
The element Protactinium was discovered by O. H. Göhring and K. Fajans in year 1913 in Germany and United Kingdom. Protactinium was first isolated by A. von Grosse in 1927.
How many valence electrons does a Protactinium atom have?
Protactinium has 5 valence electrons. Protactinium has 91 electrons out of which 5 valence electrons are present in the 5f2 6d1 7s2 outer orbitals of atom.
What is the melting Point of Protactinium?
Melting Point of Protactinium is 1845 K.
What is the boiling Point of Protactinium?
Boiling Point of Protactinium is 4273 K.
What is the melting Point of Protactinium in Kelvin?
Melting Point of Protactinium in Kelvin is 1845 K.
What is the boiling Point of Protactinium in Kelvin?
Boiling Point of Protactinium in Kelvin is 4273 K.
What is the melting Point of Protactinium in Celsius?
Melting Point of Protactinium in Celsius is 1571.85 °C.
What is the boiling Point of Protactinium in Celsius?
Boiling Point of Protactinium in Celsius is 3999.85 °C.
What is the melting Point of Protactinium in Fahrenheit?
Melting Point of Protactinium in Fahrenheit is 2861.33 °F.
What is the boiling Point of Protactinium in Fahrenheit?
Boiling Point of Protactinium in Fahrenheit is 7231.73 °F.
What is the electronic configuration of Protactinium 91?
The electronic configuration of Protactinium will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f2 6d1 7s2.
How do you write the electron configuration for Protactinium?
The electronic configuration of Protactinium will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f2 6d1 7s2.
