Periodic Table Element Comparison: Compare Elements - Phosphorus vs Cobalt
Compare Phosphorus and Cobalt
Compare Phosphorus and Cobalt on the basis of their properties, attributes and periodic table facts. Compare elements on more than 90 properties. All the elements of similar categories show a lot of similarities and differences in their chemical, atomic, physical properties and uses. These similarities and dissimilarities should be known while we study periodic table elements. You can study the detailed comparison between Phosphorus vs Cobalt with most reliable information about their properties, attributes, facts, uses etc. You can compare P vs Co on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure, Electronaffinity, physical states, electrical conductivity and many more.
Facts
| Name | Phosphorus | Cobalt |
| Atomic Number | 15 | 27 |
| Atomic Symbol | P | Co |
| Atomic Weight | 30.973761 | 58.9332 |
| Phase at STP | Solid | Solid |
| Color | Colorless | Gray |
| Metallic Classification | Other Nonmetal | Transition Metal |
| Group in Periodic Table | group 15 | group 9 |
| Group Name | nitrogen family | cobalt family |
| Period in Periodic Table | period 3 | period 4 |
| Block in Periodic Table | p -block | d -block |
| Electronic Configuration | [Ne] 3s2 3p3 | [Ar] 3d7 4s2 |
| Electronic Shell Structure (Electrons per shell) | 2, 8, 5 | 2, 8, 15, 2 |
| Melting Point | 317.3 K | 1768 K |
| Boiling Point | 553.6 K | 3200 K |
| CAS Number | CAS7723-14-0 | CAS7440-48-4 |
| Neighborhood Elements | Neighborhood Elements of Phosphorus | Neighborhood Elements of Cobalt |
History
| Name | Phosphorus | Cobalt |
| History | The element Phosphorus was discovered by H. Brand in year 1669 in Germany. Phosphorus derived its name from the Greek word phoosphoros, 'carrying light'. | The element Cobalt was discovered by G. Brandt in year 1735 in Sweden. Cobalt derived its name from the German word Kobold, meaning 'goblin'. |
| Discovery | H. Brand (1669) | G. Brandt (1735) |
| Isolated | H. Brand (1669) | G. Brandt (1735) |
Presence: Abundance in Nature and Around Us
Parts per billion (ppb) by weight / by atoms (1ppb =10^-7 %)
| Name | Phosphorus | Cobalt |
| Abundance in Universe | 7000 / 300 | 3000 / 60 |
| Abundance in Sun | 7000 / 300 | 4000 / 70 |
| Abundance in Meteorites | 1100000 / 700000 | 600000 / 200000 |
| Abundance in Earth's Crust | 1000000 / 700000 | 30000 / 10000 |
| Abundance in Oceans | 70 / 14 | 0.08 / 0.008 |
| Abundance in Humans | 11000000 / 2200000 | 20 / 2 |
Crystal Structure and Atomic Structure
| Name | Phosphorus | Cobalt |
| Atomic Volume | 16.991 cm3/mol | 6.62 cm3/mol |
| Atomic Radius | 98 pm | 152 pm |
| Covalent Radius | 106 pm | 126 pm |
| Van der Waals Radius | 180 pm | - |
| Atomic Spectrum - Spectral Lines | ||
| Emission Spectrum | Not available |  |
| Absorption Spectrum |  |  |
| Lattice Constant | 1145, 550.3, 1126.1 pm | 250.71, 250.71, 406.95 pm |
| Lattice Angle | 1.25384, 1.57725, 1.24896 | π/2, π/2, 2 π/3 |
| Space Group Name | P-1 | P63/mmc |
| Space Group Number | 2 | 194 |
| Crystal Structure | Simple Triclinic 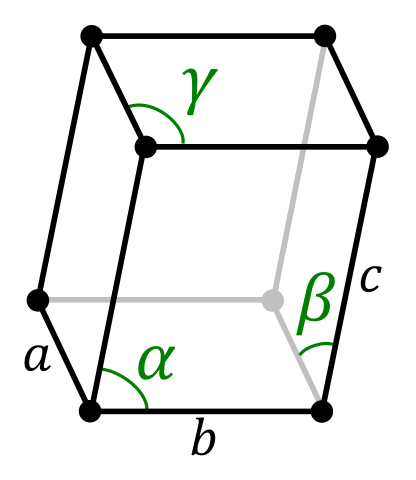 | Simple Hexagonal 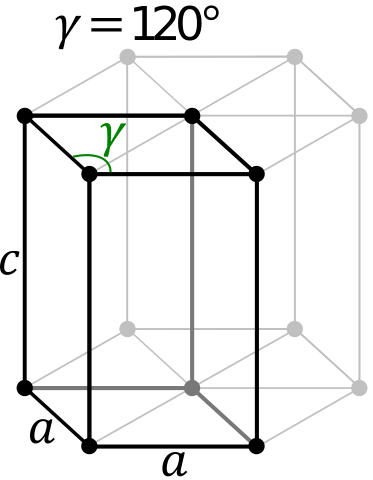 |
Atomic and Orbital Properties
| Name | Phosphorus | Cobalt |
| Atomic Number | 15 | 27 |
| Number of Electrons (with no charge) | 15 | 27 |
| Number of Protons | 15 | 27 |
| Mass Number | 30.973761 | 58.9332 |
| Number of Neutrons | 16 | 32 |
| Shell structure (Electrons per energy level) | 2, 8, 5 | 2, 8, 15, 2 |
| Electron Configuration | [Ne] 3s2 3p3 | [Ar] 3d7 4s2 |
| Valence Electrons | 3s2 3p3 | 3d7 4s2 |
| Oxidation State | -3, 3, 5 | 2, 3 |
| Atomic Term Symbol (Quantum Numbers) | 4S3/2 | 4F9/2 |
| Shell structure | 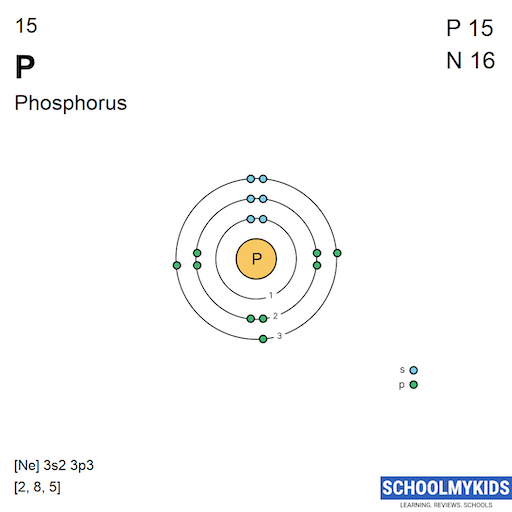 | 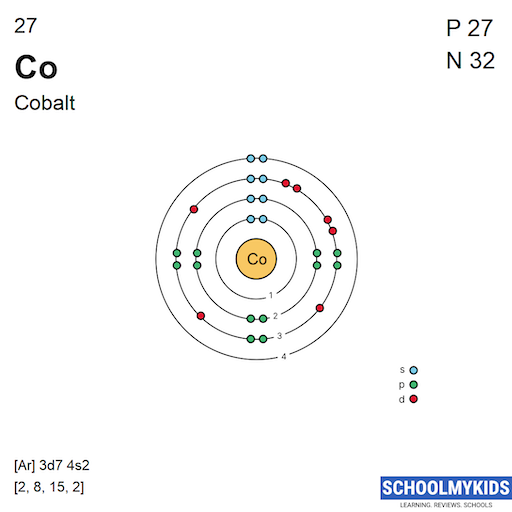 |
Isotopes and Nuclear Properties
Phosphorus has 1 stable naturally occuring isotopes while Cobalt has 1 stable naturally occuring isotopes.
| Name | Phosphorus | Cobalt |
| Known Isotopes | 24P, 25P, 26P, 27P, 28P, 29P, 30P, 31P, 32P, 33P, 34P, 35P, 36P, 37P, 38P, 39P, 40P, 41P, 42P, 43P, 44P, 45P, 46P | 47Co, 48Co, 49Co, 50Co, 51Co, 52Co, 53Co, 54Co, 55Co, 56Co, 57Co, 58Co, 59Co, 60Co, 61Co, 62Co, 63Co, 64Co, 65Co, 66Co, 67Co, 68Co, 69Co, 70Co, 71Co, 72Co, 73Co, 74Co, 75Co |
| Stable Isotopes | Naturally occurring stable isotopes: 31P | Naturally occurring stable isotopes: 59Co |
| Neutron Cross Section | 0.18 | 37.2 |
| Neutron Mass Absorption | 0.0002 | 0.021 |
Chemical Properties: Ionization Energies and electron affinity
| Name | Phosphorus | Cobalt |
| Valence or Valency | 5 | 4 |
| Electronegativity | 2.19 Pauling Scale | 1.88 Pauling Scale |
| Electron Affinity | 72 kJ/mol | 63.7 kJ/mol |
| Ionization Energies | 1st: 1011.8 kJ/mol 2nd: 1907 kJ/mol 3rd: 2914.1 kJ/mol 4th: 4963.6 kJ/mol 5th: 6273.9 kJ/mol 6th: 21267 kJ/mol 7th: 25431 kJ/mol 8th: 29872 kJ/mol 9th: 35905 kJ/mol 10th: 40950 kJ/mol 11th: 46261 kJ/mol 12th: 54110 kJ/mol 13th: 59024 kJ/mol 14th: 271791 kJ/mol 15th: 296195 kJ/mol | 1st: 760.4 kJ/mol 2nd: 1648 kJ/mol 3rd: 3232 kJ/mol 4th: 4950 kJ/mol 5th: 7670 kJ/mol 6th: 9840 kJ/mol 7th: 12440 kJ/mol 8th: 15230 kJ/mol 9th: 17959 kJ/mol 10th: 26570 kJ/mol 11th: 29400 kJ/mol 12th: 32400 kJ/mol 13th: 36600 kJ/mol 14th: 39700 kJ/mol 15th: 42800 kJ/mol 16th: 49396 kJ/mol 17th: 52737 kJ/mol 18th: 134810 kJ/mol 19th: 145170 kJ/mol 20th: 154700 kJ/mol 21st: 167400 kJ/mol 22nd: 178100 kJ/mol 23rd: 189300 kJ/mol 24th: 204500 kJ/mol 25th: 214100 kJ/mol 26th: 920870 kJ/mol 27th: 966023 kJ/mol |
Physical Properties
| Name | Phosphorus | Cobalt |
| Density | 1.823 g/cm3 | 8.9 g/cm3 |
| Molar Volume | 16.991 cm3/mol | 6.62 cm3/mol |
Elastic Properties | ||
| Young Modulus | - | 209 |
| Shear Modulus | - | 75 GPa |
| Bulk Modulus | 11 GPa | 180 GPa |
| Poisson Ratio | - | 0.31 |
Hardness - Tests to Measure of Hardness of Element | ||
| Mohs Hardness | - | 5 MPa |
| Vickers Hardness | - | 1043 MPa |
| Brinell Hardness | - | 700 MPa |
Electrical Properties | ||
| Electrical Conductivity | 10000000 S/m | 17000000 S/m |
| Resistivity | 1e-7 m Ω | 6e-8 m Ω |
| Superconducting Point | - | - |
Heat and Conduction Properties | ||
| Thermal Conductivity | 0.236 W/(m K) | 100 W/(m K) |
| Thermal Expansion | - | 0.000013 /K |
Magnetic Properties | ||
| Magnetic Type | Diamagnetic | Ferromagnetic |
| Curie Point | - | 1394 K |
| Mass Magnetic Susceptibility | -1.13e-8 m3/kg | - |
| Molar Magnetic Susceptibility | -3.5e-10 m3/mol | - |
| Volume Magnetic Susceptibility | -0.0000206 | - |
Optical Properties | ||
| Refractive Index | 1.001212 | - |
Acoustic Properties | ||
| Speed of Sound | - | 4720 m/s |
Thermal Properties - Enthalpies and thermodynamics
| Name | Phosphorus | Cobalt |
| Melting Point | 317.3 K | 1768 K |
| Boiling Point | 553.6 K | 3200 K |
| Critical Temperature | - | - |
| Superconducting Point | - | - |
Enthalpies | ||
| Heat of Fusion | 0.64 kJ/mol | 16.2 kJ/mol |
| Heat of Vaporization | 12.4 kJ/mol | 375 kJ/mol |
| Heat of Combustion | - | - |
Regulatory and Health - Health and Safety Parameters and Guidelines
| Name | Phosphorus | Cobalt |
| CAS Number | CAS7723-14-0 | CAS7440-48-4 |
| RTECS Number | {N/A, RTECSTH3495000, RTECSTH3500000, N/A} | RTECSGF8750000 |
| DOT Hazard Class | 4.1 | 4.1 |
| DOT Numbers | {1338, 1381, 2447} | 3089 |
| EU Number | - | - |
| NFPA Fire Rating | 4 | - |
| NFPA Health Rating | 4 | - |
| NFPA Reactivity Rating | 2 | - |
| NFPA Hazards | - | - |
| AutoIgnition Point | - | - |
| Flashpoint | - | - |


